Cprotons and neutrons 2. Which two particles are the primary determinants of an elements atomic mass.
These make up the core of a particleWe can refer to them as nucleons.

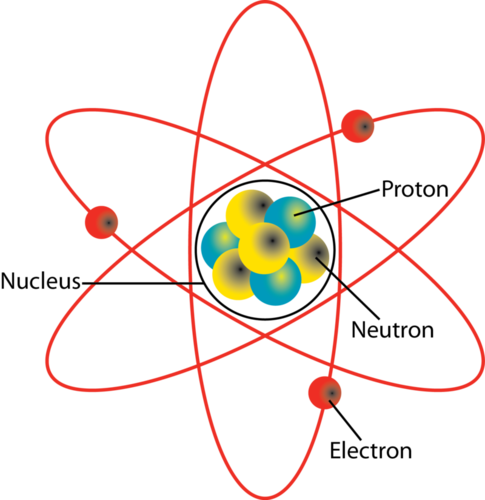
. The protons have a positive electrical charge and the neutrons have no electrical charge. There are equal numbers of protons and electrons in the atom. Two subatomic particles in the nucleus of any atom are protons and neutrons except hydrogen atom with only 1 proton no neutron.
A third type of subatomic particle electrons move around the nucleus. What Are The Two Main Regions Of An AtomAn atom is composed of two regions. Identify the subatomic particles that are found orbiting the nucleus of the atom.
A third type of subatomic particle electrons move around the nucleus. In fact the mass number of an element is the sum of its protons and neutrons. Protons and neutrons 4.
A protons B electrons C neutrons. A protons B neutrons C electrons. The protons have a net positive charge the molecules core is positive in charge overall and the electrons negative in charge rotate around the concentrated center.
Electrons are not a part of the nucleus and they revolve around it in fixed paths known as orbits. Protons are particles with a positive charge while neutrons have no charge. The subatomic particles of protons and neutrons are found in the nucleus of an atom.
Correct option is C Protons and Neutrons are part of the nucleus. Proton and neutron C. These electrons may be removed from or gained by an atom to form ions.
Hydrogen _____ Atoms always have one proton. Protons and neutrons are the two subatomic particles located in the nucleus of an atom. They make up a majority of the mass of an atom.
Which two subatomic particles are located in the nucleus of an. A proton is one of three main particles that make up the atom. The table below gives the atomic mass and relative abundance values for the three isotopes of element M.
A few points detailing the discovery and the properties of electrons are listed below. Which two subatomic particles are located in the nucleus of an atom. The nucleus of an atom is made up of two subatomic particles.
The nucleus which is in the center of the atom and contains protons and neutrons and the outer region of the atom which holds its electrons in orbit around the nucleusWhat are the two main regions in an atom What is th. The protons have a positive electrical charge and the neutrons have no electrical charge. The proton and the neutron.
Electrons of different atoms come together to participate in chemical bonding. The nucleus consists of protons and neutrons. 1 Which two particles are found in the nucleus of an atom.
The nucleus contains two types of subatomic particles protons and neutrons. Which subatomic particles contribute the most to the mass an atom. Proton and neutron Protons and neutrons are found in the nucleus of an atom.
Identify the subatomic particles that are found within the nucleus of the atom. Protons and neutrons are the two particles found inside the nucleus. They are collectively called nucleons.
Subatomic particles called_____can be found at various distances from the nucleus. The two particles found in the nucleus of an atom are neutrons and protons. Protons neutrons and electrons B.
Two subatomic particles in the nucleus of any atom are protons and neutrons except hydrogen atom with only 1 proton no neutron. The other two particles are the neutron and electron. 1 What two particles are found in the nucleus of an atom.
This is a tiny dense region at the center of the atom. Every atom has a specific set of identical protons and identical neutrons. Electron and proton 3.
4 Which particles move around. Rutherford discovered this in his gold foil. Electrons revolve around the nucleus in a particular shell.
Two amino acids are brought together to form a dipeptide. Electron and neutron D. Electrons are found in a cloud floating around the atom.
Which of the following are the subatomic particles found in the nucleus of an atom. This is an example of an _____ reaction. Protons are found in the nucleus of the atom.
Since protons have a positive charge and neutrons are neutral the nucleus of an atom is electrically positive. Electrons which have a negative charge are particles that can found orbiting outside the nucleus of an atom. 2 Which two particles are found in the nucleus of an atom A neutrons and electrons B protons and electrons c protons and neutrons d neutrons and atoms.
The nucleus contains two types of subatomic particles protons and neutrons. 3 Which subatomic particles are found in the nucleus of an atom of beryllium and what is the net charge of the nucleus. Protons and electrons D.
Which of the subatomic particles are found on the outside of the. Electrons are the subatomic particles that revolve around the nucleus of an atom. The nucleus contains two types of subatomic particles protons and neutrons.
What Two Particles Are Found In The Nucleus Of An Atom Socratic
What Are The Subatomic Particles In The Nucleus Quora

Which Two Subatomic Particles Are Located In The Nucleus Of An Atom Brainly Com
0 Comments